Ligament and Tendon Injuries
At our hospital, we provide treatment for ligament and tendon injuries using mesenchymal stem cells derived from the patient’s own adipose (fat) tissue.
For this treatment, we have formulated a “Type II Regenerative Medicine Plan,” which has been reviewed by the Certified Regenerative Medicine Committee and submitted to the Minister of Health, Labour and Welfare.
Regenerative Medicine Plan Provision Number: PB5240088
Regenerative Medicine Using Mesenchymal Stem Cells
Stem Cell Therapy for Ligament, Tendon, and Muscle Injuries
* All regenerative medicine treatments for ligament, tendon, and muscle injuries using mesenchymal stem cells derived from the patient’s own adipose tissue are provided as self-funded (out-of-pocket) medical care.
What Are Mesenchymal Stem Cells?
Mesenchymal stem cells are cells that exist throughout the body, including in bone marrow and fat tissue, and have the ability to differentiate into various types of cells.
In particular, adipose tissue allows for the efficient collection of a large number of stem cells. Because they can be harvested from subcutaneous fat with minimal damage or burden to the body, they are widely used in regenerative medicine.
What Is Stem Cell Therapy for Ligament, Tendon, and Muscle Injuries?

01Trusted Specialist in Charge
Careful consultation and treatment by Dr. Minoru Yoneda, selected as a “Best Doctor.

Clinical Advisor
Minoru Yoneda,MD
- Doctor of Medicine (PhD)
- Board-Certified Orthopedic Surgeon, Japanese Orthopaedic Association & Japanese Board of Medical Specialties
- Certified Sports Doctor, Japan Medical Association
- Certified Physician in Orthopaedic Rehabilitation Medicine, Japanese Orthopaedic Association

Career History
- July 1996
Chief, Department of Orthopedic Surgery, Osaka Welfare Pension Hospital (currently JCHO Osaka Hospital) - May 2003
Clinical Professor, Faculty of Medicine, Osaka City University - April 2009
Clinical Professor, Faculty of Medicine, Osaka University - October 2015
Specially Appointed Professor, Department of Orthopedic Surgery, Nippon Medical School
Academic Society Memberships
- Honorary Member, Japanese Orthopaedic Society for Sports Medicine
- Honorary Member, Japanese Society for Surgery of the Shoulder
- Representative, Japan Shoulder Arthroscopy Research Society
- Representative, Osaka Shoulder Arthroscopy Forum
- Advisor, Sports Medicine Forum
- ISAKOS (International Society of Arthroscopy, Knee Surgery and Orthopaedic Sports Medicine): Honorary Member
- French Society of Arthroscopy: Honorary Member
- Korean Shoulder and Elbow Society: Honorary Member
Awards & Honors
- 1990
Academic Award, 16th Annual Meeting of the Japanese Shoulder Society - 1998
AOA International Traveling Fellow, American Orthopaedic Association - 1998
Academic Award (Mentorship), 24th Annual Meeting of the Japanese Shoulder Society - 2006
Academic & Medical Award, Osaka Welfare Pension Hospital - 2015
Academic Award (Mentorship), 42nd Annual Meeting of the Japanese Shoulder Society - 2015
Alumni Award, Department of Orthopedic Surgery, Nippon Medical School - 2020
Distinguished Service Award, Japanese Orthopaedic Association - Best Doctors, The Best Doctors in Japan 2024–2025
- Doctor of Doctors Network – Distinguished Specialist Clinician
02In-House Cell Culturing
Within our group, we have established a system that manages the entire process consistently — from harvesting to processing and administration. This enables us to provide treatment with stable quality and ensured safety.

03Team-Based Medical Practice
In addition to stem cell administration, patients receive periodic imaging examinations such as 3-Tesla MRI scans, along with appropriate exercise guidance provided by rehabilitation staff, based on the imaging results.
About Consultations
Book a Consultation
Consultation
Appointment Required
Monday to Friday, 8:30 – 17:00
For Appointments & Inquiries
ISEIKAI International General Hospital
Orthopedic Surgery Department
international-division@holonicsystem.com
Treatment Process
1) Explanation of Treatment
- The doctor and medical staff will carefully explain the treatment.
- If you wish to proceed after the explanation, you will be asked to sign a consent form.
After that, a detailed treatment schedule will be arranged.

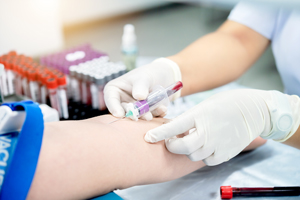
2) Pre-Fat Harvesting Examination
- Before treatment, you will undergo a blood test, including screening for viruses and infectious diseases.
3) Fat Tissue Harvesting
- Performed as an outpatient (same-day) procedure.
- Fat is collected from an inconspicuous area of the abdomen.
- The harvested fat tissue is immediately handed over to a clinical cell culture specialist on site.
*One week after harvesting, the wound condition will be checked.

4) Evaluation of Motor Function & Exercise Guidance
- A physical therapist or occupational therapist will conduct an assessment of motor function and provide appropriate exercise guidance.

Transport of Tissue and Cell Culturing
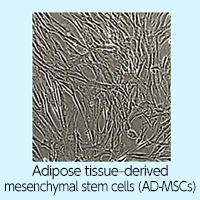
- The harvested tissue is transported by clinical cell culture specialists to our affiliated cell processing facility in Osaka City (Osaka ISEIKAI Cancer and Neurodegenerative Disease Treatment Clinic).
- At the cell processing facility, mesenchymal stem cells are isolated from the adipose tissue and cultured until the required number of cells for treatment (150 million to 300 million) is reached.

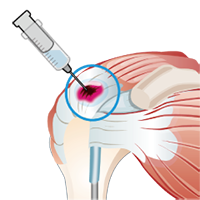
6) Cell Administration
- On the day of administration, the mesenchymal stem cells cultured at the processing facility are prepared and transported according to the treatment schedule.
- The transported mesenchymal stem cells are then administered to the affected treatment site in the Department of Orthopedic Surgery.
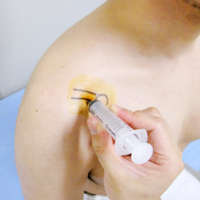
7) Post-Treatment Follow-Up
- After cell administration, regular follow-up consultations and MRI scans are conducted.
(Typical schedule: 3 weeks, 6 weeks, 3 months, and 6 months after cell administration — total of 4 visits)
Cost
Consultation fees
Blood tests
Fat tissue harvesting
Medications (such as antibiotics)
Stem cell culturing and expansion
Stem cell administration and related equipment
Please contact us for pricing information.
Please note: MRI scans and other imaging tests performed during follow-up consultations after stem cell administration are considered self-funded treatments and will incur additional charges.
Treatment Effects果
By reducing inflammation, this therapy is expected to promote the healing and regeneration of damaged tissues.
Since your own cells are used, there is no risk of rejection.
Side Effects of Treatment
Because this therapy uses your own fat-derived stem cells, the likelihood of allergic reactions or rejection is considered extremely low.
However, as the procedure involves injections, it is not possible to completely eliminate the risk of infection or other complications.
To minimize such risks, we implement strict hygiene control and infection prevention measures.
Before treatment, we will provide a detailed explanation. Please feel free to ask us any questions or share any concerns you may have.
Patients Who Are Not Eligible for This Treatment
- Patients with hypersensitivity to anesthetics used during tissue collection or substances used in the manufacturing process
- Patients with a history of allergic reactions to antibiotics used during cell culturing
- Patients who test positive for viral or bacterial infections (e.g., HIV, syphilis, etc.)
- Underage patients
- Pregnant or breastfeeding patients
- Patients diagnosed with cancer
- Patients with severe obesity (BMI ≥ 40)
- Patients found to have abnormalities during preoperative coagulation tests