Orthopedic Surgery
Our hospital is officially approved by the Ministry of Health, Labour and Welfare (MHLW) of Japan to provide regenerative treatment using autologous adipose-derived mesenchymal stem cells (MSCs) for ligament and tendon injuries.
Regenerative Medicine Provision Plan Number: PB5240088
Approval Date: January 28, 2025
Mesenchymal Stem Cell Therapy
Regenerative Medicine Using Autologous Adipose-Derived Mesenchymal Stem Cells for Ligament and Tendon Injuries
* All treatments using adipose-derived MSCs for ligament and tendon injuries are offered as self-funded (private) care.
Overview of the Treatment
This treatment involves injecting stem cells extracted from adipose tissue into damaged ligaments or tendons to aid in the repair of the injured areas. It helps alleviate pain, improve mobility, and reduce joint instability. Additionally, stem cells are known to have anti-inflammatory effects, which are expected to contribute to pain relief.
What Are Mesenchymal Stem Cells (MSCs)?
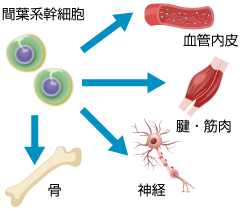
MSCs are multipotent cells found throughout the body, including in bone marrow and adipose tissue. They can differentiate into various cell types. Adipose tissue, in particular, is an efficient and rich source of stem cells. Since collection from subcutaneous fat is minimally invasive, it is commonly used in regenerative therapies.
What Is Regenerative Therapy for Ligament and Tendon Injuries?
Cultured mesenchymal stem cells are injected into injured ligaments, tendons, or muscles. The treatment promotes tissue regeneration and repair, reduces inflammation, and improves symptoms such as pain, restricted movement, and joint instability.
①Expert-led Care You Can Trust
Dr. Minoru Yoneda, a Best Doctors-selected orthopedic surgeon, provides precise treatment based on extensive experience.
Clinical Advisor : Dr. Minoru Yoneda
- Doctor of Medicine
- Board-certified Orthopedic Specialist (Japanese Orthopaedic Association)
- Certified Sports Doctor (Japan Medical Association)
- Certified Musculoskeletal Rehabilitation Specialist (JOA)

Career Highlights
- July, 1996 : Former Chief, Orthopedic Surgery, Osaka Welfare Pension Hospital (now JCHO Osaka)
- May, 2003 : Former Clinical Professor, Osaka City University
- April, 2009 : Former Clinical Professor, Osaka University
- October, 2015 : Visiting Professor, Nippon Medical School (Orthopedic Surgery)
Professional Affiliations and Honors
- Honorary Member, Japanese Society of Shoulder and Elbow Surgery
- Honorary Member, ISAKOS, French Society of Arthroscopy, Korean Shoulder and Elbow Society
- Multiple academic awards including Best Doctors Japan 2024–2025
②Safe and Reliable In-House Cell Culturing
Cells are processed in our GMP-compliant cell processing facility.

③Team-Based Multidisciplinary Care
Treatment involves not only stem cell injections, but also 3T MRI scans and individualized exercise guidance from rehabilitation staff based on imaging assessments.
About medical examination
1.Booking a Consultation
Consultation Days
By Appointment Only
Please contact us by phone for appointments
For Appointments and Inquiries
ISEIKAI International General Hospital
Orthopedic Department
2.Treatment Flow
-
1) Initial Consultation
- Doctors and medical staff provide detailed explanations.
- If the patient wishes to proceed, they sign a consent form and the schedule is set.

-
2) Preoperative Testing
- Blood tests (including virus/infection screening)
- Physical/occupational therapy assessment of motor function
-
3) Fat Tissue Collection
- Performed in a day surgery room.
- Tissue is collected from a discreet area of the abdomen and immediately handed to the clinical cell culture team.

-
4) Transport and Cell Culturing
- Tissue is transported to our internal GMP-grade cell processing facility in Osaka.(Osaka Iseikai Cancer and Neurological Intractable Disease Treatment Clinic)
- MSCs are isolated and cultured to obtain 100–300 million cells as needed.
Stem cell expansion culture
Day 0
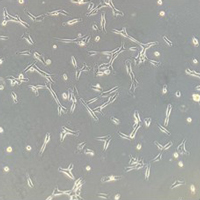
Day 3
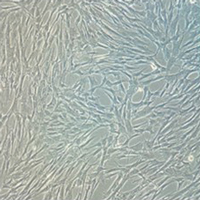
Day 5
-
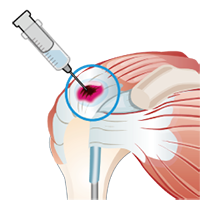
5) Stem Cell Injection
- On the day of injection, cultured MSCs are delivered and administered into the treatment site at the outpatient clinic.
-
6) Post-Treatment Follow-Up
- Regular checkups including MRI scans at 3 weeks, 6 weeks, 3 months, and 6 months post-injection.
2.Cost
Consultation Fee
Blood Test
Fat Tissue Collection
Antibiotics and Painkillers
Stem Cell Culturing and Expansion
Stem Cell Injection and Support Bracing
1,100,000 Yen(tax included)
* Additional charges apply for MRI and other diagnostics during post-treatment checkups, as these are not included in the package.
3.Expected Benefits
MSCs cultured from your own abdominal fat can be injected into injured areas to help suppress inflammation and promote natural tissue regeneration. Because your own cells are used, there is no risk of immune rejection.
4.Potential Side Effects
While clinical studies have reported minor side effects such as injection site pain or mild infections, no serious adverse effects requiring treatment or resulting in long-term complications have been observed.
5.Who Is Ineligible for This Treatment?
- Patients with allergies to anesthetics or materials used in cell preparation
- History of allergic reactions to antibiotics used in culturing
- Positive results in viral/bacterial tests (e.g., HIV, syphilis)
- Minors
- Pregnant or breastfeeding women
- Cancer patients
- Patients with a BMI over 40 (morbid obesity)
- Patients with abnormal coagulation test results

